Principal Investigators:



3959 John Pappajohn Pavilion, 200 Hawkins Drive
Iowa City, IA 52242
Tel: (319) 356-3214
Email: yusuf-menda@uiowa.edu
Overview
The number of persons diagnosed with neuroendocrine tumors (NETs) has risen dramatically in the United States. Iowa NET SPORE researchers are probing novel targeted therapies that deliver a killing blow to tumor cells while sparing normal tissues, investigating the impact of increasingly used diabetes and obesity drugs (incretin mimetics like GLP-1 receptor agonists) on NET growth, and conducting clinical trials to improve patient outcomes. The proposed approaches and therapies should be applicable to other chemotherapy resistant and/or immunologically “cold” cancers, such as prostate and breast, as well as tumors that express high levels of receptors for the incretin mimetics.
The primary objective of this SPORE is to provide scientific advances that inform more personalized comprehensive care of NET patients that will improve the length and quality of their lives. This will be achieved through four overarching goals:
- Support innovative translational research in NETs through three new projects
- Project 1 investigates a new strategy to sensitize pancreatic NETs (pNET) to immune checkpoint inhibitor therapy using unique mouse pNET models and a Phase 1b trial in pNET patients.
- Project 2 combines pre-clinical metabolic studies of radiation-induced lipid peroxidation with a novel Phase 1 trial of 212Pb Pentixather targeting C-X-C Receptor 4 (CXCR4) in lung NETs and NECs.
- Project 3 defines the tumor promoting potential and receptor dependence of commonly used GLP1 and GIP receptor agonists in gastroenteropancreatic NETs, thereby informing patient risks with these drugs.
- Provide support to translational investigators through interactive cores
- Administrative Core facilitates communication and collaboration between projects, cores, collaborators.
- Biospecimens Core provides access to multiple sources of rare NET tissue and guides their scientific use while providing accurate tumor classification for all specimens.
- Clinical Core provides access to data in the NET Registry on >2,400 patients eager to participate in trials and provides clinical trial coordination as well as regulatory support to projects, DRP, and CEP.
- Biostatistics Core provides rigorous experiment and trial design, data analysis, quality control and biostatistics education.
- Enlist and encourage new translational researchers in NETs through developmental research and career enhancement programs that successfully transition awardees to independent NET funding.
- Promote translational research in NETs through horizontal and vertical collaborations that result in innovative treatments and strong evidence-based guidelines for patients with NETs and NECs.
Projects
- Project 1: Improving immunotherapy in pancreatic NETs
-
Project Co-Leaders:
Dawn Quelle, PhD (Basic Co-Leader)
Steven K. Libutti, MD (Clinical Co-Leader; Director, Rutgers Cancer Institute)
This novel multi-institutional study addresses the critical need for new therapeutic options, potentially curative, for metastatic pancreatic NETs (pNETs). The role of B and/or plasma cells in pNET biology is unknown, but in other types of tumors their presence is associated with better patient survival and improved response to immune checkpoint inhibitor (ICI) therapy. This project will determine the importance of B/plasma cell tumor infiltrates in pNET biology and patient outcomes while testing, for the first time, if induction of B/plasma cell tumor infiltration by cyclin-dependent kinase 4/6 (CDK4/6) blockade enhances antitumor immunity and efficacy of ICI therapy in pNETs. Findings from novel mouse pNET models and a window of opportunity trial in pNET patients may define a new strategy to sensitize pNETs to ICI agents, thereby offering the promise of sustained anti-tumor activity, extended patient survival, and potential cure.
- Project 2: Targeting CXCR4 and Redox Metabolism for Alpha Particle Therapy of Pulmonary Neuroendocrine Tumors and Carcinomas
-
Project Co-Leaders:
Douglas Spitz, PhD (Basic Co-Leader)
Yusuf Menda, MD (Clinical Co-Leader)Atypical carcinoids of the lung and lung neuroendocrine carcinomas (NECs) are currently incurable with most patients succumbing to disease within five years after diagnosis. This Project will pursue the development of an exciting new treatment paradigm for these cancers based on high LET alpha radioligand therapy (RLT) with 212Pb Pentixather targeting the CXCR4 receptor combined with cancer cell specific manipulations of metabolic oxidative stress. The studies will optimize clinical imaging, therapy delivery, and dosimetry techniques and, if successful, will have lasting impact on improving outcomes for neuroendocrine lung cancer patients.


- Project 3: GLP-1R/GIPR agonists in promoting gastroenteropancreatic (GEP) NET progression
-
Project Co-Leaders:
Po Hien Ear, PhD (Basic Co-Leader)
Joseph Dillon, MD (Clinical Co-Leader)
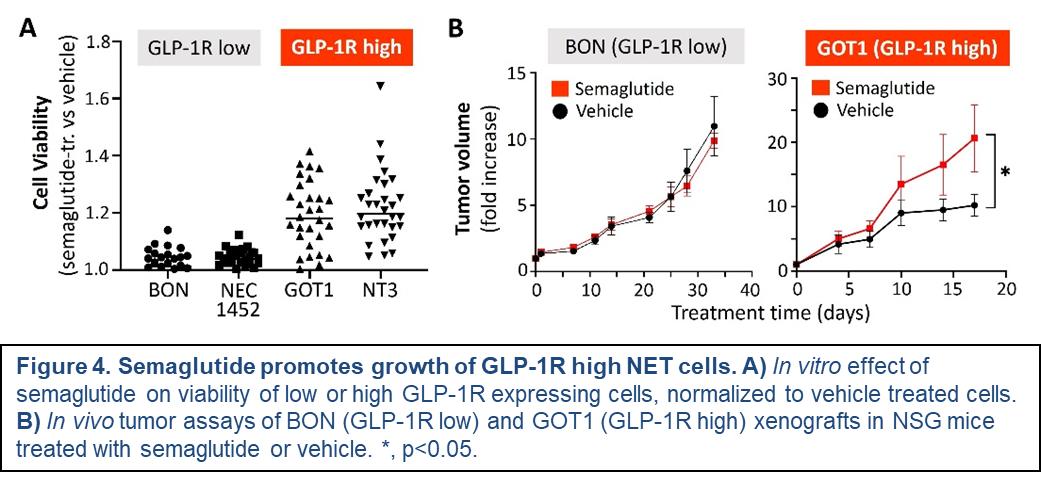
James Howe, MD (Clinical Co-Leader)This timely investigation will define the role and impact of increasingly used obesity and diabetes drugs, GLP-1 and GIP receptor agonists like Ozempic, on GEPNET growth. Up to 12% of Americans are already taking these drugs (known as incretin mimetics) and that number is only expected to rise. The central hypothesis of this project is that GLP-1R and GIPR agonists selectively promote GEPNET growth depending on expression levels of their targeted receptors. This project will be the first to 1) determine the predicted risks of incretin mimetic therapy in patients with GEPNETs that express high levels of these receptors, 2) define the biological impact, mechanisms of action, and receptor dependence of these drugs in human and mouse GEPNET models, and 3) test the possibility that current NET-approved therapies could be beneficial in blocking the tumor-promoting effects of these agents. Results should identify which NET patients may be at risk from incretin mimetic therapy, which incretin mimetic may be taken safely, and how unwanted incretin mimetic effects might be ameliorated.
Administrative Core
Core Directors:
Dawn Quelle, PhD
James Howe, MD
Yusuf Menda, MD
The Administrative Core provides the main organizational structure and oversight of all SPORE activities. The overall goal is to support innovative, collaborative, and rigorous research in NETs and oversee the translation of our team’s findings into improved diagnostic and therapeutic options for NET patients.
Biospecimens Core
Core Directors:
Andrew Bellizzi, MD
Michael O’Rorke, PhD
The Biospecimens Core provides a coordinated, centralized, and dedicated service for the procurement, processing, and annotation of high quality biospecimens from patients in our Iowa NET Registry and also from SEER Residual and Iowa Virtual Tissue Repositories. The specimens collected and maintained herein constitute the largest and most well-characterized resource of NET and NEC tumor tissue in the United States. This work is fundamental for investigators from each main Project, Developmental Research Program, and Career Enhancement Program to adequately tackle clinically relevant scientific questions relating to these perplexing tumors.
Biostatistics Core
Core Directors:
Patrick Breheny, PhD
Gideon Zamba, PhD
Robust biostatistical support is essential for the Iowa Neuroendocrine Tumor (NET) SPORE to conduct rigorous and meaningful translational NET research. The Biostatistics Core provides this expertise in several ways: 1) through full integration of Biostatistics into project / clinical trial study design, implementation, execution, analysis, publication, and grant development; 2) through synergistic facilitation of shared research outcomes among the various project and core groups of the NET SPORE; and 3) through career development and NET investigator training in biostatistics.
Clinical Core
Core Directors:
Joseph Dillon, MD
Muhammad Furqan, MD
The overarching goal of the Clinical Core is to serve as a bidirectional link between patients with neuroendocrine tumor (NET) or neuroendocrine carcinoma (NEC) and advances in research, diagnosis, treatment, long-term care and, ultimately, disease prevention. Toward this end, 98% of patients attending the Iowa NET Clinic participate in the IRB-approved NET Registry, which now includes over 2,400 subjects who can be directly contacted if a clinical trial is being developed for which the patient might qualify. We are also leading a multi-institutional collaboration to develop a de-identified database of over 7,000 subjects that will enable population science and patient reported outcomes research.
Developmental Research Program (DRP)
Program Directors:
Yusuf Menda, MDDawn Quelle, PhD
Our DRP seeks to bring new ideas and innovative techniques to bear on the diagnosis, curative therapy, and ultimately, the prevention of NETs. It is anticipated that support of developmental research projects will continue to result in the generation of new hypotheses that will be tested in full SPORE-sponsored projects or through peer reviewed external grant support. The long-term goal of the Iowa NET SPORE program is to translate the findings generated by developmental projects into an improved length and quality of life for patients with neuroendocrine tumors.
Career Enhancement Program (CEP)
Program Directors:
James Howe, MD
Dawn Quelle, PhD
The goal of the CEP is to guide the development of creative translational scientists who are experienced in multidisciplinary research for future leadership in NET research. The provision of ample opportunities for training and career enhancement is a top priority of the University of Iowa NET SPORE and scientific community. The CEP is a critical component of our long-term commitment to recruit bright, energetic new investigators into NET translational cancer research, thereby infusing the field with innovative talent and ideas.
